Our ImmunoSafe-HLA I and ImmunoSafe-HLA II panels are me- ticulously designed to enhance the predictivity of your preclinical assays. With 10 HLA Class I- or HLA Class II-matched donors, these panels can be tailored to suit your specific assay requirements. By providing a comprehensive resource to mimic diverse immuno- logical responses, they empower researchers to achieve more ac- curate and relevant experimental outcomes. Their versatility and adaptability ensure seamless integration into existing research protocols, making them invaluable tools for deciphering the complexities of the immune system and driving groundbreaking discoveries in biomedical research.
| Prospective collection of specific subsets of PBMC* | ||
|---|---|---|
| OFFER NO. | PRODUCT | SPECIFICATION |
| 133 94113 010 | Human PBMC, healthy donor, ImmunoSafe-HLA1 | Cryo, 10 × 106 cells/vial |
| 133 94113 020 | Human PBMC, healthy donor, ImmunoSafe-HLA1 | Cryo, 20 × 106 cells/vial |
| 134 94114 010 | Human PBMC, healthy donor, ImmunoSafe-HLA2 | Cryo, 10 × 106 cells/vial |
| 134 94114 020 | Human PBMC, healthy donor, ImmunoSafe-HLA2 | Cryo, 20 × 106 cells/vial |
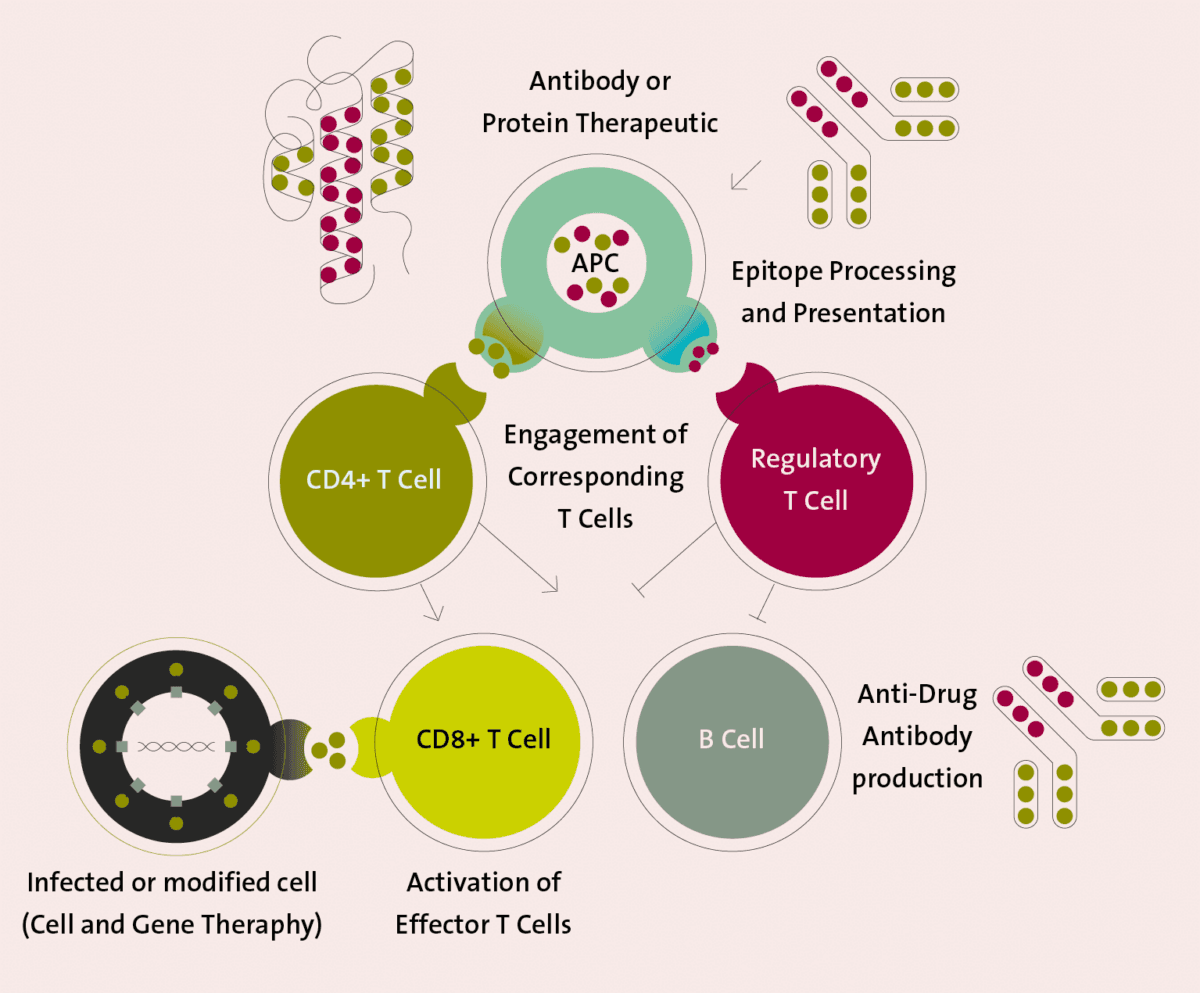
The evaluation of the immunogenicity risk of novel protein therapeutics is pivotal for guaranteeing their safety and efficacy, and it is subject to several challenges including variations in immunogenic response between different populations, the change in the immunogenicity of vaccines with age, and the general unsuitability of animal models in predicting immune responses in humans. Immunogenicity may be a desired (wanted) or undesired (unwanted) effect in novel protein therapeutics: Wanted Immunogenicity is a central aspect of vaccine efficacy, as the injection of the desired antigen stimulates an immune response against it, thus creating a protection against the active pathogen Unwanted immunogenicity constitutes the undesired immune response against protein therapeutics, often resulting in the production of anti-drug-antibodies (ADAs) with inactivating or neutralizing properties against the therapeutic agent. Because of the potential unwanted effects of immunogenicity, regulators expect that developers of non-vaccine biological therapeutics employ validated immunogenicity assays to detect and characterize the risk of ADAs formation in the clinical development phases.